
Companies importing and manufacturing LED lighting products for the EU market must ensure compliance with various regulations and directives. These, in turn, set requirements concerning electrical safety, energy efficiency, labelling, documentation, and lab testing.
This guide explains how different directives and regulations are relevant to LED lighting products, including the Low Voltage Directive, Ecodesign Directive, the Energy Labelling Framework Regulation, and the RoHS Directive.
Continue reading LED Lighting Regulations in the European Union: An Overview




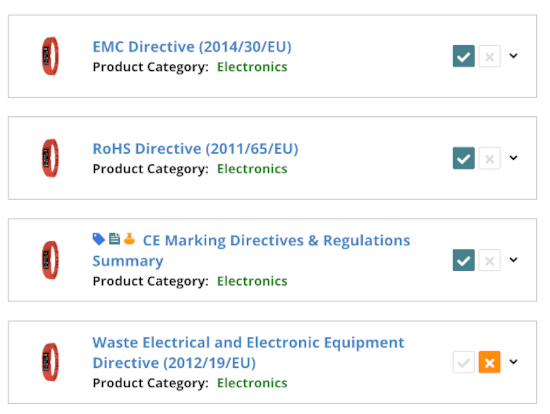








 The Medical Devices Regulation sets requirements according to device class. More specifically, medical devices are defined as class I, IIa, IIb, or III. In turn, this determines certain requirements, and which conformity assessment procedure the manufacturer must follow.
The Medical Devices Regulation sets requirements according to device class. More specifically, medical devices are defined as class I, IIa, IIb, or III. In turn, this determines certain requirements, and which conformity assessment procedure the manufacturer must follow.

