The Medical Devices Regulation (MDR) regulates medical devices and their accessories manufactured or imported for the European Union market.
This guide provides examples of products covered by the Medical Devices Regulation, explains which medical devices require a notified body, and elaborates on various requirements for such products including documentation, labelling, and testing.
Content Overview

FREE CONSULTATION CALL (30 MIN)
 Ask questions about compliance requirements
Ask questions about compliance requirements Countries/markets:
Countries/markets:
 Learn how we can help your business
Learn how we can help your business
You will speak with:Ivan Malloci or John Vinod Khiatani
What is the Medical Devices Regulation?
The Medical Devices Regulation contains requirements for medical devices for human use and their accessories imported or manufactured for the EU market.
Its main goal is to protect both the patients and users of the devices. In order to achieve this objective, the regulation sets conformity assessment procedures and requirements aiming at ensuring the safety, quality and performance of covered devices.
Ensuring compliance can, in practice, mean that you have to take the following steps:
1. Identify harmonised standards and implement these into your technical drawings
2. Submit prototypes for testing
3. Create a Declaration of Conformity
4. Apply CE mark and other lablling
5. Create technical documentation
6. Create user instructions
7. Identify the applicable conformity assessment process
8. Identify the applicable device class
9. Submit products and documents to a notified body
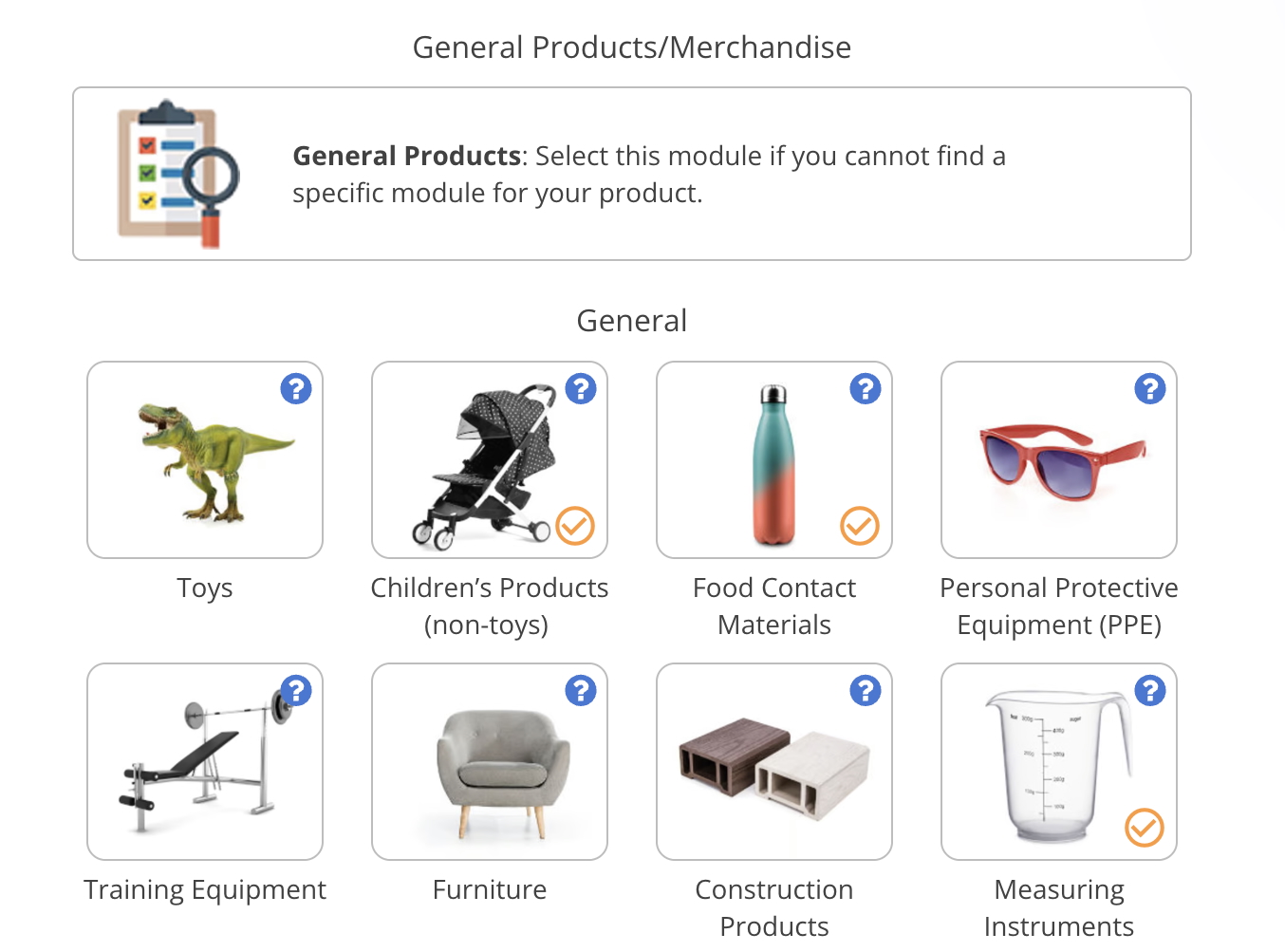
What products are covered by the Medical Devices Regulation?
The Medical Devices Regulation covers the following products:
- Medical devices
- Accessories for medical devices
- Groups of products without an intended purpose are listed in Annex XVI of the regulation
Medical devices are articles intended for human beings for one or more medical purposes mentioned in the Medical Devices Regulation. The following products are also treated as medical devices:
- Products for the control or support of conception
- Products specifically intended for the cleaning, disinfection or sterilisation of medical devices
Examples of products that are medical devices include:
- Corrective glasses and lenses
- Hospital beds
- Walking aids
- Sticking plasters
- Band-aids
- Examination gloves
- Scalpels and scalpel handles
- Examination lamps
- Electronic thermometers
- IPL/LED/Laser equipment for hair removal only
How can I determine the class of a medical device?
The Medical Devices Regulation requires that medical devices be classified into the following classes:
- Class I devices
- Class IIa devices
- Class IIb devices
- Class III devices
Generally speaking, the higher the class, the higher the invasivity of the medical device and the longer its intended use on human bodies. Annex VIII of the Medical Devices Regulation contains the classification rules. Additionally, the European Commission has published a guide on the application of the classification rules.
To access the guide, enter into the European Commission’s site containing guidance documents concerning the Medical Devices Regulation and look for the document entitled “Guidance on classification of medical devices”.
To use the guide, go to the section entitled “4.2 General explanation of rules/practical issues/examples” to find an explanation for and a provision of product examples which would be covered by each classification rule.
What are the safety and performance requirements?
Medical devices must meet the general and safety requirements set out in Annex I to the Medical Devices Regulation. The Annex contains topics relating to:
a. The establishment of a risk management system
b. Design and manufacture of the product
c. Information supplied with the device
What harmonised standards apply to medical devices?
Harmonised standards refer to standards that are adopted under the Medical Devices Regulation, the references of which have been published in the Official Journal of the European Union. If applied, they give rise to a presumption of conformity with the requirements of the Medical Devices Regulation covered by those standards.
The following are examples of harmonised standards under the Regulation:
a. EN 285 – Sterilization – Steam sterilizers – Large sterilizers
b. EN 455-3 – Medical gloves for single use – Part 3: Requirements and testing for biological evaluation
c. EN ISO 10993-10 – Biological evaluation of medical devices – Part 10: Tests for skin sensitization
d. EN IEC 60601-2-83 – Medical electrical equipment – Part 2-83: Particular requirements for the basic safety and essential performance of home light therapy equipment
Conformity assessment procedures
The Medical Devices Regulation requires medical devices to undergo a conformity assessment procedure to ensure the product’s compliance with the relevant requirements. Article 52 of the regulation specifies, for each class, the articles or Annex(es) that should be observed.
| Requirements | Article 52(7) | Annex IX | Annex X | Annex XI |
|
Scope |
Class I |
Class I (sterile products/ products with a measuring function/ reusable surgical instruments) Class IIa Class IIb Class III |
Class IIb Class III |
Class I (sterile products/ products with a measuring function/ reusable surgical instruments) Class IIa Class IIb Class III |
|
Notified body’s identification number label |
/ |
Yes |
Yes |
Yes |
|
Documentation |
a. Declaration of conformity b. Technical Documentation |
a. Documentation related to the quality management system b. Declaration of conformity c. Documentation related to the manufacturer’s post-market surveillance system and, where applicable, on the PMCF plan d. Documentation related to the clinical evaluation plan e. Technical documentation f. Quality control reports and test data |
a. Technical documentation b. Clinical evaluation report c. EU type-examination certificate |
a. Certificate under Part A or B of Annex XI (Production quality assurance or product verification respectively) b. Declaration of conformity c. EU type-examination certificates |
|
Notified body |
/ |
Yes |
Yes |
Yes |
Note that, in some cases, the requirements of more than an annex must be followed in order to complete the conformity assessment procedure. For example, for Class III devices one can choose between applying the conformity assessment procedure contained on:
- Annex IX, or
- Annex X and Annex XI
Documentation
In this section, we list documentation mentioned in the Medical Devices Regulation and that is typically required for medical devices and provide descriptions for each of them.
| Document Type | Description |
| Declaration of Conformity | The Declaration of Conformity is a document that states that the requirements of the Medical Devices Regulation have been fulfilled concerning the covered device. The required content of the document can be found in Annex IV. |
| Technical documentation | The technical documentation must contain the details necessary to demonstrate that the medical device conforms to the requirements of the regulation.
The contents of the technical documentation are contained in Annex II and Annex III. Examples of its content include:
|
| Instructions | Medical devices must be accompanied by the information needed to identify the device and its manufacturer, and by any safety and performance information. Requirements can be found in Chapter III in Annex I. |
| Test reports | The Medical Devices Regulation requires test reports to demonstrate conformity with its requirements. Test reports are provided by lab testing companies. |
| Implant card | An implant card is required to be delivered with implantable devices. Contents of the implant card are contained in Article 18 of the Medical Devices Regulation. |
| Quality system documentation | If manufacturers utilise a notified body’s services, they might need to provide quality system documentation (e.g., quality programmes, plans, manuals, and records). |
| Notified Body documents | A notified body involved in a conformity assessment procedure should draw up an evaluation report, and an EU-type examination certificate if the product complies with all necessary requirements. |
Management and surveillance
The Medical Devices Regulation requires the establishment of the following systems:
a. A quality management system addressing the aspects contained in Article 10(9) of the Medical Devices Regulation.
b. A risk management system as described in Chapter I in Annex I of the Medical Devices Regulation.
c. A post-market surveillance system
Labelling

Below we list the labelling requirements contained in the Medical Devices Regulation.
| Label item | Description |
| CE marking | Medical devices require CE marking. The CE marking must be affixed to the device or its sterile packaging, in any instructions for use and on any sales packaging. |
| Traceability Information | The following information for the purposes of traceability is required:
|
| Unique Device Identification (UDI) | The UDI allows for identification and facilitates the traceability of medical devices. Requirements concerning the UDI are contained in Article 27 of the Medical Devices Regulation.
A list of UDI issuing entities designated to provide manufacturers with a list of UDIs to assign to medical devices can be found here. |
Claims
The Medical Devices Regulation prohibits the use of text, names, trademarks, pictures and figurative or other signs that may mislead the user concerning the device’s intended purpose, safety and performance.
Registration
Manufacturers should assign a UDI and register their device. The registration should be done via the European database on medical devices (EUDAMED).
The guidance page “UDI/Devices registration” published on the EU website contains information on the registration process.
Lab testing
Manufacturers of medical devices need to have their products lab tested to prove that their products comply with the Medical Devices Regulation’s requirements, as well as the relevant harmonised standards.
After the product has passed testing, manufacturers should receive a test report indicating that the medical device complies with the relevant requirements of the regulation.
For some medical devices whose conformity assessment procedure necessarily involves a notified body, one of the tasks of the notified body would be to assess the testing of the product according to relevant technical specifications under the Medical Devices Regulation.
Test methods
Test methods can be found be found in standards. For instance, EN 455-3 – Medical gloves for single use – Part 3: Requirements and testing for biological evaluation employs:
a. Immunological methods for the measurements of natural rubber latex allergens, and
b. Methods for the determination of aqueous extractable proteins in natural rubber gloves using the modified Lowry assay
Lab testing companies
The following are a few lab testing companies that have testing services for medical devices:
- Eurofins
- TÜV Rheinland
- Nelson Laboratories
- Intertek
- TÜV SÜD
Notified bodies
EU Notified bodies can be found through the NANDOS database. To search for notified bodies for medical devices, follow the steps below:
1. Go to the NANDOS database
2. Click the link entitled “Notified bodies”
3. Click the link entitled “Search by legislation”
4. Under the column “Legislation name” find the link entitled “Regulation (EU) 2017/745 on medical devices”
The following are examples of notified bodies that can be found using the database:
- TÜV NORD CERT GmbH (Germany)
- National Standards Authority of Ireland (NSAI) (Ireland)
- IMQ ISTITUTO ITALIANO DEL MARCHIO DI QUALITÀ S.P.A. (Italy)
- CENTRO NACIONAL DE CERTIFICACION DE PRODUCTOS SANITARIOS (Spain)
- DEKRA Certification B.V. (Netherlands)



















.png)
.png)
.png)