Vapes and electronic cigarettes sold in the European Union are subject to various regulations and directives that concern electrical safety, battery safety, and e-liquids.
This guide explains what importers and manufacturers must know about the Tobacco Products Directive, the Batteries Regulation, REACH, and other relevant compliance requirements.
Content Overview

FREE CONSULTATION CALL (30 MIN)
 Ask questions about compliance requirements
Ask questions about compliance requirements Countries/markets:
Countries/markets:
 Learn how we can help your business
Learn how we can help your business
You will speak with:Ivan Malloci or John Vinod Khiatani
Tobacco Products Directive
The Tobacco Products Directive sets requirements for e-cigarettes, e-liquids, and other tobacco and related products. The directive defines an “electronic cigarette” as a product that contains a consumable nicotine vapour. It can be disposable, refillable (via a refill container), or rechargeable (with single-use cartridges).
Importers and manufacturers of e-cigarettes and e-liquids should:
a. Ensure children cannot tamper or open the products
b. Ensure the ingredients are of high purity
c. Submit an electronic notification regarding the products they sell
d. Provide safety and health information
e. List the ingredients on the packaging
f. Provide a leaflet containing instructions and information on harmful effects, risk groups, toxicity, and product addictiveness
Note that Article 4 sets ISO standards to evaluate emissions. However, these requirements are only listed in the context of “tobacco products”. Thus, we are not certain if they apply to e-cigarettes, which are not mentioned in the article or standard titles.
Ingredients
The directive sets ingredient restrictions for e-cigarettes and other related products. Here are a few examples:
a. Nicotine-containing liquid in refill containers should not exceed 10 ml
b. Nicotine in disposable single-use cartridges should not exceed 2 ml
c. Nicotine-containing liquid should not contain more than 20 mg/ml of nicotine
d. Nicotine-containing liquid should contain high-purity ingredients
e. E-cigarettes should not contain additives with emission-colouring properties
r. E-cigarettes should not contain additives that aid in the inhalation of nicotine
Physical requirements
E-cigarettes and their refill containers should have a leak-proof refill mechanism, and be:
- Child-proof
- Tamper-proof
- Protected against leakage and breakage
Notification requirements
Importers and manufacturers of e-cigarettes and refill containers should submit a notification of novel tobacco products to the EU country where they sell their product, via the EU Common Entry Gate.
They should include the following information in the notification:
- Importer or manufacturer name and contact details
- List of all ingredients and emissions, by brand name, type, and quantities
- Toxicological information about the ingredients and emissions referring to consumer’s health
- Nicotine dosage and uptake information regarding normal usage conditions
- Product component description, including opening and refill mechanisms
- Description of the production process, with a conformity declaration
- The importer’s and manufacturer’s declaration of responsibility
Documentation requirements
Importers and manufacturers should ensure that their e-cigarettes and refill containers include an information leaflet that contains the following information:
- Usage and storage instructions
- Contra-indications
- Warnings for specific risk groups
- Possible adverse effects
- Addictiveness and toxicity
- Contact details of the importer or manufacturer
- Contact details of natural or legal contact person in the EU
Labelling requirements
Importers and manufacturers should make sure that the unit packets and the outside packaging, or the e-cigarettes and their refill containers include the following information:
- A product ingredient list ordered by weight
- The nicotine content of the product
- Nicotine delivery per dose
- Batch number
- Recommendation to keep the product away from children
- Nicotine flavourings
If the product contains nicotine, the unit packets and the outside packaging should also bear one of the following health warnings:
“This product contains nicotine which is a highly addictive substance. It is not recommended for use by non-smokers”
Or
“This product contains nicotine which is a highly addictive substance.”

Batteries Regulation
The Batteries Regulation sets battery requirements covering the following areas:
- Sustainability
- Safety
- Labelling
- Documentation
- Extended producer responsibility
The regulation covers all categories of batteries, including batteries that are incorporated into products, such as e-cigarettes and vapes.
Standards
We could not find any harmonised standards specific to batteries for e-cigarettes. Instead, we list some examples of standards that are relevant to lithium batteries and batteries in general.
EN IEC 60086-1 – Primary batteries – Part 1: General
This standard specifies:
- Requirements for primary cells and batteries
- Requirements for the standardisation of batteries
- Standard test methods for testing primary cells and batteries
- System letters
- Electrodes
- Electrolytes
- Nominal and maximum open circuit voltage of electrochemical systems
prEN IEC 60086-2-2 – Primary batteries – Part 2: Physical and electrical specifications of lithium batteries
This standard covers primary batteries and focuses on the physical and electrical specifications of lithium batteries.
Note that this standard is still under draft and its status indicates “committee stage”.
EN IEC 60086-4 – Primary batteries – Part 4: Safety of lithium batteries
This standard covers primary lithium batteries and specifies safety requirements and tests to ensure those batteries can be safely used under normal use and abuse conditions.
Documentation
Importers and manufacturers of e-cigarettes are required to provide documentation, such as:
- Technical documentation
- Declaration of Conformity
- Battery usage, removal, and replacement instructions
- Battery usage, removal, and replacement safety information
- Test reports
Labelling requirements

The regulation requires you to label your batteries. Here are several examples of information you should label your batteries with:
a. CE marking
b. Capacity information for rechargeable batteries
c. Minimum average duration for non-rechargeable batteries
d. The separate collection symbol per Part B of Annex VI
e. The chemical symbol Cd or Pb for batteries containing more than 0.002% of cadmium or 0.004% of lead
f. A QR code containing:
- A copy of the Declaration of Conformity
- A report on battery due diligence policy
- Information on the prevention and management of waste batteries
RoHS Directive
The RoHS Directive restricts the manufacture and usage of certain chemicals and heavy metals in electronic products in the EU. It additionally sets CE marking, labelling, documentation, and testing requirements.
Substance restrictions
The directive establishes maximum concentration values, by weight, for these substances:
- Lead < 0.1%
- Mercury < 0.1%
- Cadmium < 0.01%
- Hexavalent chromium < 0.1%
- Polybrominated biphenyls (PBB) < 0.1%
- Polybrominated diphenyl ethers (PBDE) < 0.1%
- Bis(2-Ethylhexyl) phthalate (DEHP) < 0.1%
- Benzyl butyl phthalate (BBP) < 0.1%
- Dibutyl phthalate (DBP) < 0.1%
- Diisobutyl phthalate (DIBP) < 0.1%
Documentation
Importers and manufacturers of e-cigarettes should provide the following documentation:
- Declaration of Conformity
- Technical documentation (e.g. product description, harmonised standards used)
- Test report
Labelling requirements
You should label your e-cigarette and packaging with the following information:
- CE marking
- Product traceability information (e.g. batch, serial number, name, contact details)
EMC Directive
The Electromagnetic Compatibility (EMC) Directive sets electromagnetic compatibility requirements for electronic and electric equipment.
The directive covers a wide range of electrical and electronic products, which may include e-cigarettes. Note that the directive does not cover “inherently benign equipment”, which includes batteries if they don’t have active electronic circuits.
Documentation
You should provide the following documentation:
- Declaration of Conformity
- User instructions
- Technical documentation
- Test reports
Labelling requirements
You should label your e-cigarette and packaging with:
- CE marking
- Product traceability label (e.g. serial number, manufacturer name, contact details)
Low Voltage Directive
The Low Voltage Directive sets safety requirements regarding electrical equipment with an input or output voltage rating of 50–1000V for alternating current and 75–1500V for direct current.
Therefore, it applies to external chargers, which generally have an input voltage higher than 50V (e.g. 220V).
You should ensure your external charger:
- Is manufactured according to good engineering practice
- Does not harm the consumer’s health and safety
- Conforms to requirements in relevant harmonised standards
Documentation
Importers and manufacturers of external chargers should provide:
- Declaration of Conformity
- Technical documentation
- User instructions
- Test reports
Labelling requirements
You should label your external charger, as well as the packaging where applicable, with the following:
- CE marking
- Product traceability information (e.g. name, address, product serial number)
WEEE Directive
The Waste Electrical and Electronic Equipment (WEEE) Directive covers electronic and electric equipment, including e-cigarettes. It sets registration, reporting, and collection requirements. Note that a waste management company can fulfil these responsibilities for you. The directive also sets labelling requirements.
Labelling requirements
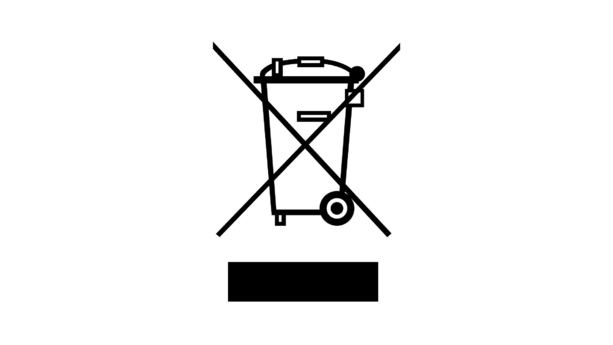
You should label your product with the separate collection symbol displayed above. If the product is too small, you should add the label in the packaging, and instructions for use.
Registration
Producers should register in the EU countries where they sell their electronic products. Annex X states that the following registration information must be provided:
- Producer’s name, address, and contact details
- Producer’s national identification code
- Category of EEE
- Type of EEE
- Brand name
- How the producer meets its EPR responsibilities
- Selling technique (e.g. distance selling)
- Statement declaring that the provided information is factual
Reporting
Producers should submit the following reporting information to the EU countries in which they sell their products:
a. The producer’s national identification code
b. Reporting period
c. Category of EEE
d. Quantity, by weight, of EEE placed on the national market
e. Quantity, by weight, of EEE that is separately collected, disposed of, recycled, recovered, and prepared for re-use, within the EU country or outside the EU
Collection
Producers are also responsible for collecting their products. This is generally done by contracting a waste management company, which can take care of the collection, in exchange for a fee.
REACH
The REACH Regulation sets substance restrictions, including when these substances are used in consumer products. The restrictions apply, for instance, to the plastic and metal components of e-cigarettes and their mouthpieces. E-liquids may also contain substances that are restricted by REACH.
Annex XVII
Annex XVII to REACH lists substances that either have restrictions or are banned from use in consumer products. E-cigarettes, for example, may contain plastic components, or other components that include restricted substances, such as the following:
- Phthalates (e.g. DEHP, DBP, BBP, DIBP) – 0.1% by weight
- Lead and its compounds – 0.005% by weight
- Cadmium and its compounds – 0.01% by weight of plastic material
Substances of Very High Concern (SVHC)
Substances of Very High Concern (SVHC) are deemed to have permanent and negative effects on human health and the environment. REACH regulates substances on the SVHC Candidate List. These substances may be:
- Carcinogenic, mutagenic, or reprotoxic (CMR)
- Persistent, bioaccumulative, and toxic (PBT)
- Endocrine-issue causing
Just because a substance is on the SVHC Candidate List does not mean it is prohibited. If you use more than 0.1% w/w of a listed substance in your product, you should use SCIP to notify ECHA of the substance’s SVHC content.
Here are a few examples of SVHCs that may be relevant to e-cigarettes:
- Formaldehyde
- Acrylaldehyde
- Benzene
- Lead
- Nickel
EU Member States Regulations
Some EU member states set additional regulations covering e-cigarette and e-liquid products, on top of the Tobacco Products Directive. Here are several examples of countries and their e-cigarette laws:
a. France – Decree n° 2016-1117 of August 11, 2016, on the manufacture, display, sale and use of tobacco products, vaping products and products for smoking made from plants other than tobacco
b. Belgium – Royal Order of October 28, 2016, on the manufacture and sale of E-cigarettes
c. Germany – Tobacco Duty Modernization Act, 2021
d. Spain – Royal Decree 579/2017, of June 9, regulating certain aspects related to the manufacture, presentation and marketing of tobacco products and related products
e. Sweden – Law (2018:2088) on tobacco and similar products
f. Netherlands – Tobacco and Smoking Products Act
Lab Testing
You generally need to have your products tested to prove product safety and compliance with relevant regulations and directives. For example, you should ensure that your e-cigarettes adhere to chemical, electrical, and emission safety rules.
When your product passes lab testing, you receive a test report proving compliance with the requirements of the relevant regulations and directives. Note that, while some regulations may not explicitly require a test report, in practice testing may still be needed, for example, to ensure that you comply with relevant substance restrictions.
Here we provide more information on the tests that are required for the regulations listed in this guide.
| Regulation | Lab testing |
| Tobacco Products Directive | The directive sets various substance restrictions, for instance on nicotine and additives. Thus, it is important to test e-liquids to ensure that you comply with the requirements.
Additionally, you must test your product to make sure that it is child-proof, tamper-proof, and leak-proof. Finally, testing may also be necessary to evaluate properties such as emissions, nicotine dosage, and more. Note that this information is necessary to fulfil the notification requirements. |
| Batteries Regulation | The regulation requires you to have your batteries undergo testing to ensure they comply with requirements, such as:
|
| RoHS Directive | The directive sets restrictions on several substances (e.g. heavy metals and phthalates), so you need to have your e-cigarette tested to ensure the substances do not exceed the set maximum concentration values. |
| EMC Directive | The directive requires you to have your equipment tested to ensure it meets essential requirements such as the following:
a. The electromagnetic disturbance generated by your equipment should not negatively affect the operation of other devices b. Your equipment should handle the expected level of electromagnetic interference during its intended use without losing its functionality |
| Low Voltage Directive | Importers and manufacturers should generally test their electrical equipment, such as external chargers, to ensure compliance and device safety.
For instance, according to Annex I, electrical equipment should:
|
| REACH | The regulation sets substance restrictions and lists them in Annex XVII and the SVHC Candidate List. You should ensure that your e-cigarettes and e-liquids comply with such requirements:
Here are three examples of substances and their restrictions by weight:
|
E-cigarettes testing companies
Here are a few companies that offer to test e-cigarettes and e-liquid products:
- Eurofins
- Labstat
- Tofwerk
- SMTLab
Additional Requirements
In this section, we list some additional requirements that are also relevant to e-cigarettes, for example, due to substance restrictions or battery transport safety.
| Regulation | Description |
| CLP Regulation | This regulation sets classification, labelling, and packaging requirements for substances, which also include substances that can be found in e-cigarettes, such as nicotine. |
| General Product Safety Regulation | This regulation requires consumer products, such as e-cigarettes, to be safe prior to their sale.
It covers, for example, electrical and mechanical safety of products that aren’t already covered by other regulations and directives, such as the Low Voltage Directive. |
| Inland Transport of Dangerous Goods Directive | This directive requires the transport of lithium batteries to adhere to the requirements of the International Carriage of Dangerous Goods by Road (ADR), which in turn mandates that lithium batteries comply with UN 38.3 requirements. |
| Persistent Organic Pollutants Regulation | This regulation sets restrictions for several substances, such as PFAS, that can be used in e-cigarettes, and their components. |
Recommended articles






















.png)
.png)
.png)




We are new manufacturer of vaping device, now we need to know the standard of product/finish good device quality testing, before release product to market. Such as the quality items need to be checked i.e. nicotine e-liquid content, gas leakage detection, inhaling resistant check so on.
Please support for it since have plan to sold out our product to EU and USA market.
no mention of nicotine free products….what is the legislation on moving product from, say, uk to portugal…what import restrictions exist in this notoriously nanny country?
Hi, do you able to arrange full legalization and commercialization in one of EU country?
Thank you for your article. It will definitely help me a lot…
I want to ask the following questions:
Do disposable vapes need CE marking ?
What are the import duties for disposable vapes in Europe?
(small package,500 puffs>,and 2ml tank>
Thank you
As a professional I worked in consumer products more than 16 years, I recommend COMPLIANCEGATE to peopel who need it. This is very professional website.
It gives very detailed compliance rules for junior individual or corporate.
Even for a simple CE marking, most of people don’t understand it deeply.
Thank you very much for the kind words Chris!