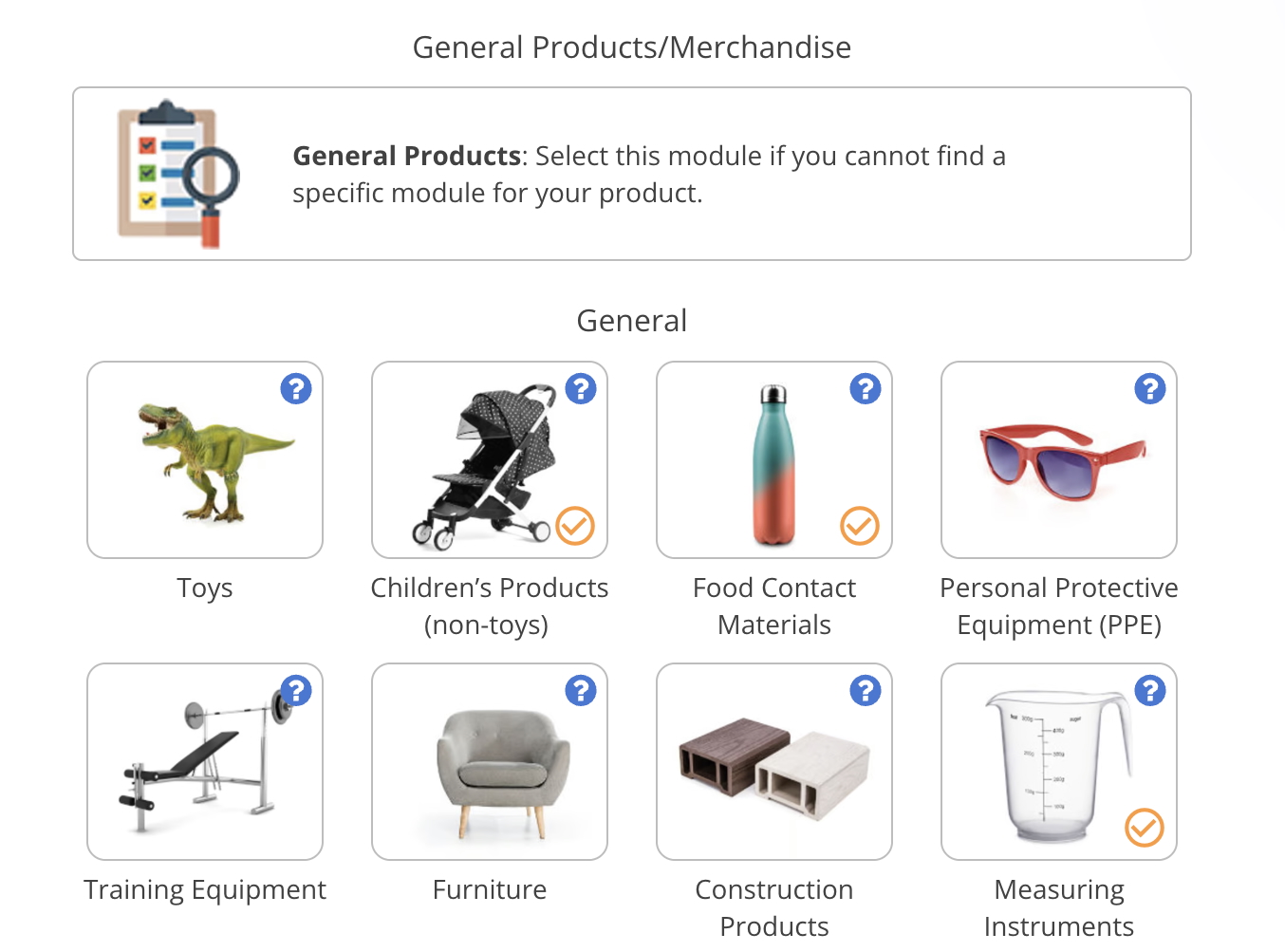
Annex XVII of the REACH regulation sets restrictions for a wide range of substances, including phthalates, lead, cadmium, and nickel. Further, some substance restrictions are specific to certain product categories, such as textiles or childcare articles.
Keeping track of Annex XVII substances is important as you generally cannot sell products or mixtures that contain such substances above the set limits.
In this guide, we address common questions related to Annex XVII and present examples of substances that are restricted for use in consumer products. Additionally, we explain how new restrictions are added to the Annex.
Content Overview

FREE CONSULTATION CALL (30 MIN)
 Ask questions about compliance requirements
Ask questions about compliance requirements Countries/markets:
Countries/markets:
 Learn how we can help your business
Learn how we can help your business
You will speak with:Ivan Malloci or John Vinod Khiatani
What is Annex XVII?
Annex XVII of the REACH Regulation is a list of substances on their own, in a mixture or in an article that contains restrictions or prohibitions to be manufactured or used in the EU.
The specific restrictions may vary according to the substances and product categories. For example, Entry 27 of Annex XVII sets a weekly migration limit for the release of nickel in products coming into direct and prolonged contact with skin (e.g. jewellery).
In comparison, Entry 51 of Annex XVII restricts certain phthalates to a concentration level equal to or greater than 0.1% by weight in plasticised materials in toys and childcare articles.
What is the difference between Annex XVII and the SVHC List?
Both Annex XVII and the SVHC Candidate List belong to the REACH Regulation and list chemicals that are deemed harmful to human beings or the environment, and whose uses are regulated in the EU.
The main difference between Annex XVII and the SVHC Candidate List is the level of substance control and the specific requirements.
Upon the request of a consumer, REACH requires that manufacturers and importers selling products containing more than 0.1% by weight of substances in the SVHC Candidate List, must provide sufficient information on the safe use of the products. In this case, it is also necessary to register the substance in the SCIP database.
On another hand, substances that are listed in Annex XVII are subject to different constraints. Some substances are prohibited from being used in any context (e.g. asbestos). Other substances are limited to certain concentration levels (e.g. phthalates) or weekly migration limits (e.g. nickel) when used in certain consumer products.
If the substance content is above the set limit, then the product is non-compliant and cannot be sold.

Are Annex XVII substances banned?
Some substances are banned, such as asbestos fibres or PCT. However, some substances are restricted to a certain amount (e.g. 0.1% by weight) or a certain migration limit (e.g. 0.5 μg/cm2/week). Such substances can be used if the conditions of the restrictions are complied with.
For example, the use of nickel is restricted in body piercings unless the migration limit of the nickel release from these objects is less than 0.2 μg/cm2/week. However, for articles that are intended to have direct contact with human skin, such as a bracelet, the nickel migration limit is set to 0.5 μg/cm2/week.
Can I sell products that contain Annex XVII substances above the set limits?
Importers and manufacturers must comply with REACH Annex XVII requirements, including ensuring that the concentration level of regulated substances is kept under the prescribed limit. Thus, products that contain restricted substances above the set limitations cannot be sold in the EU.
List of Restricted Substances
Here we list some examples of restricted substances under Annex XVII.
Phthalates
Phthalates are a group of chemical substances that are often added to plastics to improve the flexibility and durability of the products. For example, Annex XVII restricts the use of the following phthalates, individually or in combination, to a maximum of 0.1% by weight in plasticized materials in toys or childcare articles:
- DEHP
- DBP
- BBP
- DIBP
Here are some examples of products that may contain restricted phthalates:
- Toys
- Childcare articles
- Putties
- Adhesives
- Furniture
- Construction materials
- Footwear
Lead and its compounds
Lead and its compounds are also listed. For example, the Annex states that they must not be used in any individual part of jewellery articles if the concentration of lead is equal to or greater than 0.05% by weight.
As another example, lead in accessible articles that can be placed in the mouths of children under reasonable conditions is also limited to 0.05% by weight.
Here are some examples of products that might contain lead:
- Bracelets
- Necklaces
- Rings
- Wristwatches
- Brooches
Nickel and its compounds
The use of nickel is restricted in body-piercing objects unless the migration limit of the nickel release from these objects is less than 0.2 μg/cm2/week.
For articles that are intended to have direct and prolonged contact with human skin, the nickel migration limit is set to 0.5 μg/cm2/week. Examples of such articles include:
- Wrist-watch straps
- Earrings
- Rings
- Necklaces
- Bracelets
- Chains
- Anklets
Cadmium and its compounds
The Annex contains several restrictions for Cadmium. For example, the maximum allowable concentration level of cadmium that is allowed to be used in specified plastics is 0.01% by weight of the plastic materials. These plastic materials include but are not limited to:
- PVC
- PUR
- LDPE
- PP
- PBT
- PET
Metal parts of jewellery that contain more than 0.01% of cadmium by weight are also prohibited also. Examples of such jewellery include:
- Bracelets
- Necklaces
- Rings
- Brooches
- Cufflinks
In addition, cadmium and its compounds are prohibited in cadmium plating metallic articles or components of the articles used in equipment and machinery for:
- Food production
- Cooling and freezing
- Printing and book-binding
- Household good
- Furniture
- Sanitary ware
Chromium VI compounds
The use of chromium VI compounds is restricted in some products, such as the following:
a. Articles that contain leather coming into contact with the skin should not contain chromium VI in concentrations equal to or greater than 0.0003% of the total dry weight of the leather
b. Hydrated cement and cement-containing mixtures should not contain more than 0.0002% soluble chromium VI of the total dry weight of the cement
Mercury
Mercury is restricted in some measuring equipment. For example, mercury is not allowed to be used in devices that are intended for sale to the public such as:
- Fever thermometers
- Manometers
- Barometers
- Sphygmomanometers
- Thermometers
These devices that are intended for industrial or professional uses are also prohibited if they contain mercury:
- Barometers
- Hygrometers
- Manometers
- Sphygmomanometers
- Tensiometers
- Thermometers and other non-electrical thermometric applications
Azocolourants and Azodyes
Azocolourants and azodyes are also listed in the Annex. Azodyes which may release aromatic amines listed in Appendix 8 of REACH in concentrations above 0.003% by weight in textile and leather articles that may come into direct and prolonged contact with human skin or oral cavity must not be sold.
Here are some examples of articles that may contain azodyes:
- Purses
- Clothing
- Bedding
- Towels
- Hairpieces
- Wigs
- Hats
- Sleeping bags
- Footwear
- Gloves
- Chair covers
Additionally, azodyes listed in Appendix 9 of REACH must not be sold or used as substances or in mixtures intended for colouring textile and leather articles in concentrations above 0.1% by weight.
Toluene
Toluene cannot be sold or used as a substance or in mixtures in a concentration equal to or above 0.1% by weight where it is intended to be used in adhesive or spray paints for consumer use.
Microplastics
Synthetic polymer microparticles (or microplastics) are listed in the Annex. There are many restrictions related to the substances. For example, they cannot be sold as substances on their own or in mixtures in a concentration equal to or above 0.01% by weight.
Additionally, the above restriction would apply to certain products in the future. Such products include:
- Rinse-off products
- Nail products
- Make-up products
- Leave-on products
- Detergents
- Waxes
- Polishes
The annex also mentions specific documentation, labelling, and testing requirements.
Formaldehyde
Formaldehyde and formaldehyde-releasing substances are listed in the Annex. There are two key effective dates for the restrictions. The following restrictions apply from 6 August 2026:
a. 0.062 mg/m3 for furniture and wood-based articles
b. 0.080 mg/m3 for articles other than furniture and wood-based articles
After 6 August 2027, the concentration of formaldehyde released in the interior of road vehicles must not exceed 0.062 mg/m3.
Is the Annex XVII list updated?
Any EU Member State, the European Commission or the European Chemicals Agency (ECHA) can propose to add substances that pose an unacceptable risk to human health or the environment to Annex XVII. If the proposal is accepted, the substance is added to the annex.
How does the restriction procedure work?
In this section, we summarise the procedure to add a substance to REACH Annex XVII.
Registry
A Member State or ECHA at the request of the European Commission or the European Commission itself may prepare a restriction proposal (a dossier). The intention to prepare a dossier is published in the ECHA’s registry of intentions until outcome to give advance notice to the public and interested parties, such as manufacturers or importers.
Proposal preparation
The dossier should be prepared according to the requirements of Annex XV and must contain information such as:
- Substance identity
- Reasons and explanations for the proposed restrictions
- Identified risks
- Information on alternatives to the substance and the costs
- Environmental and human health benefits resulting from the restriction
Consultation
If the dossier conforms to REACH Annex XV requirements, it is made publicly available for consultation and comments on the ECHA’s website. At this time, the Committee for Socio-economic Analysis (SEAC) should also prepare an opinion about the socio-economic impacts of the suggested restrictions.
Additionally, the ECHA’s Committee for Risk Assessment (RAC) evaluates whether the suggested restriction is appropriate in reducing the risk to human health or the environment based on the dossier and public comments.
Decision
The European Commission considers the RAC and SEAC’s compiled opinions and prepares a draft amendment for Annex XVII. The draft is submitted to the WTO to assess whether the restriction poses a technical barrier to international trade.
The final decision is made involving the Member States, the European Council, and the European Parliament. The decision then is published in the EU Official Journal and the restriction is published on ECHA’s site.
Enforcement
Once the restriction has been adopted, Member States must enforce the rules and require manufacturers and importers to comply with them.
How do I know which Annex XVII listed substances to test for?
Our recommendation is that you contact a testing company. When labs quote REACH testing they usually quote Annex XVII testing by default.
You may also want to clarify that they should provide a quote both for Annex XVII substances testing and SVHC testing – but keep the substance list and quotation separate.
Where can I find a complete list of Annex XVII restricted substances?
You can visit the official website of ECHA to browse the complete list of substances under Annex XVII
, which is available on the page “Substances restricted under REACH”.
Keep in mind that new substances can be added to Annex XVII from time to time. Therefore, whether you are a manufacturer or importer, you should keep track of the updates of Annex XVII.


















.png)
.png)
.png)